Technology
ISK Ltd has successfully completed a Phase I clinical trial with Nerofe, a promising new treatment for patients with progressive cancer. The trial demonstrated that Nerofe is safe for human use, with no toxic effects observed. Furthermore, Nerofe remains stable in human blood.
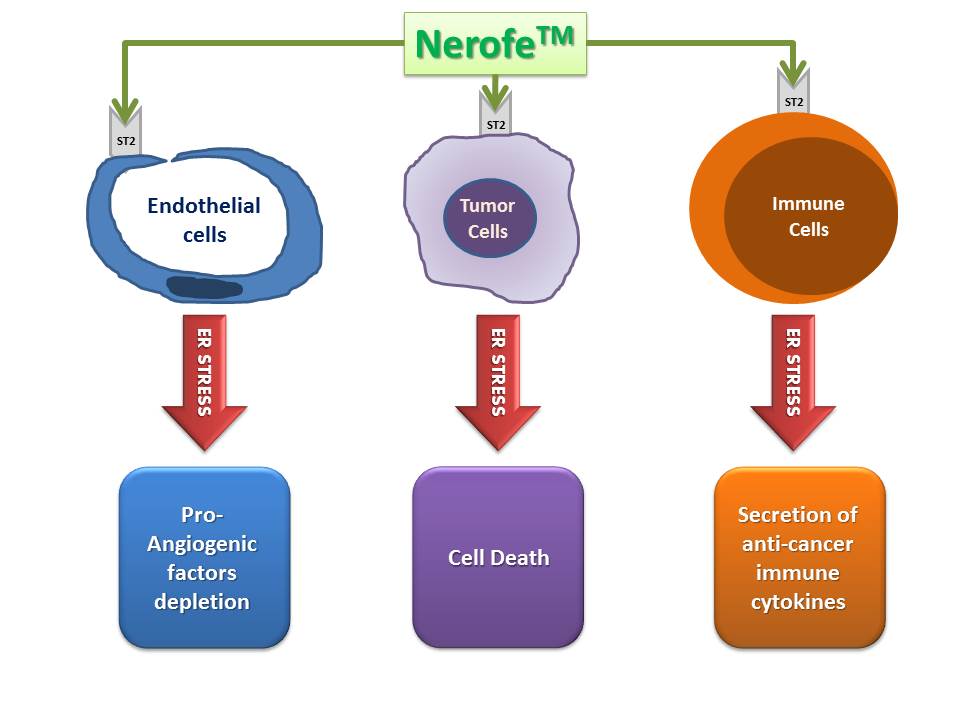
Nerofe’s Triple Action Mechanism
Unlike conventional anti-cancer drugs that target a single biological process supporting cancer progression, Nerofe is the first highly safe anti-cancer agent that inhibits all three key biological processes:
- Anti-angiogenic effect: Nerofe induces a strong anti-angiogenic effect, inhibiting the formation of blood vessels around the tumor.
- Activation of anti-cancer immune response: Nerofe activates the body’s immune response against cancer cells.
- Inhibition of cancer cell proliferation: In vitro experiments have shown that Nerofe has a direct and strong inhibitory effect on cancer cell proliferation.
Predictive Marker for Treatment Success
We have developed a novel marker that can predict the success of treatment with Nerofe. Patients whose tumor biopsies tested positive for this marker remained in the trial for six months or longer, maintaining a stable disease state.
Our Focus
ISK Ltd is primarily focused on treating patients with Acute Myeloid Leukemia (AML), pancreatic cancer, and triple-negative (TN) breast cancer.