Oncology Market Overview
The oncology market continues to grow rapidly. As of 2024, the Oncology Drugs market worldwide is expected to reach a projected revenue of US$214.10 billion1. This forecast indicates that the market is likely to experience an annual growth rate (CAGR 2024-2028) of 13.80%, resulting in a market volume of US$359.10 billion by 20281. Today, it is estimated that there are more than 32.6 million people living with cancer worldwide. The positive market dynamics reinforce and support the attractiveness of the oncology field, a sector that continues to show considerable potential compared to other therapeutic areas.
Our Progress
In 2013, we began our Phase I trials, focusing on oncology patients with progressive disease. We completed the dose escalation stage in the fourth quarter of 2014.
As of 2024, we have made significant advancements:
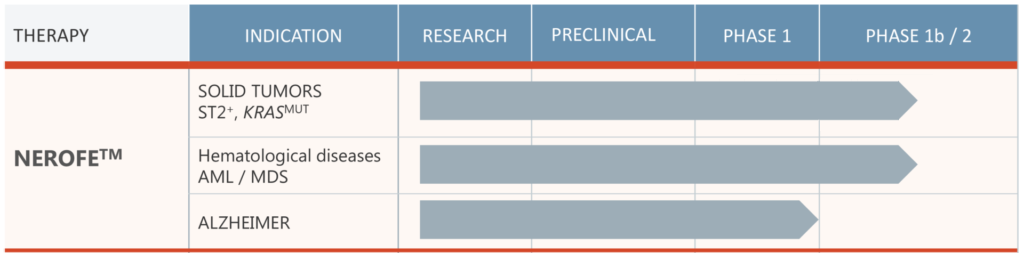
FDA Approval: We have received FDA approval for the orphan drug accelerated procedure for Acute Myeloid Leukemia (AML). This is a significant milestone in our journey to provide effective treatments for rare diseases.
Clinical Trials: We are currently conducting clinical trials in two key areas:
- ST2+ KRAS Mutated Solid Tumors: We are in Phase 1b/2a of our clinical trials for ST2+ KRAS mutated solid tumors. These trials are being conducted at Georgetown University in Washington.
- AML/MDS: We are also in Phase 1b/2a for our trials on Acute Myeloid Leukemia/Myelodysplastic Syndromes (AML/MDS). These trials are taking place at the Sylvester Comprehensive Cancer Center in Miami.
Future Plans
Following the submission of the IND/EMA equivalent for commercial assessment evaluation, we plan to develop and position Nerofe as a maintenance therapy at all ages following CR induced by induction/consolidation therapy vs placebo and 1st/2nd line treatment add-on therapy versus doctor’s choice in AML patients of any age (presumably 1st line >60 and 2nd line pretreated <60).
Other Developments
In addition to Nerofe, we have several other peptides, all derivatives of natural human proteins, which we intend to develop. At least one of them has very interesting anti-cancer properties while another one has the ability to regenerate several types of white blood cells and could be developed as supportive care following chemotherapy or radiotherapy.
We are committed to our mission of developing innovative treatments and improving patient outcomes. Stay tuned for more updates on our progress.

